Double Replacement Reaction Worksheet. Each atom is left with a complete outer shell. That’s when something will get made, changed, or destroyed. Fill the test tubes to about half full or to the height of the white plastic posts. Phet Balancing Chemical Equations Worksheet Answers.
Chemical reactions is the main focus of chemistry as a result of that’s where the motion is. That’s when one thing will get made, changed, or destroyed.

It’s important that school students work at a stage acceptable for them. Learners will write a multiplication sentence for each of the issues and use it to multiply numbers in equal teams. Start studying hand lettering at current with our free Dual Brush Pen letter tracing practice worksheets.
Double Alternative Response Lab Copper Sulfate & Ammonium Hydroxide
It is nice for the first day of college or to rejoice Read Across America. Oh the locations youll go writing activity is great for a variety …

Force & energy is what decides if the reaction happens and how fast. Mathematics helps you keep an inventory of all of the starting and ending materials.
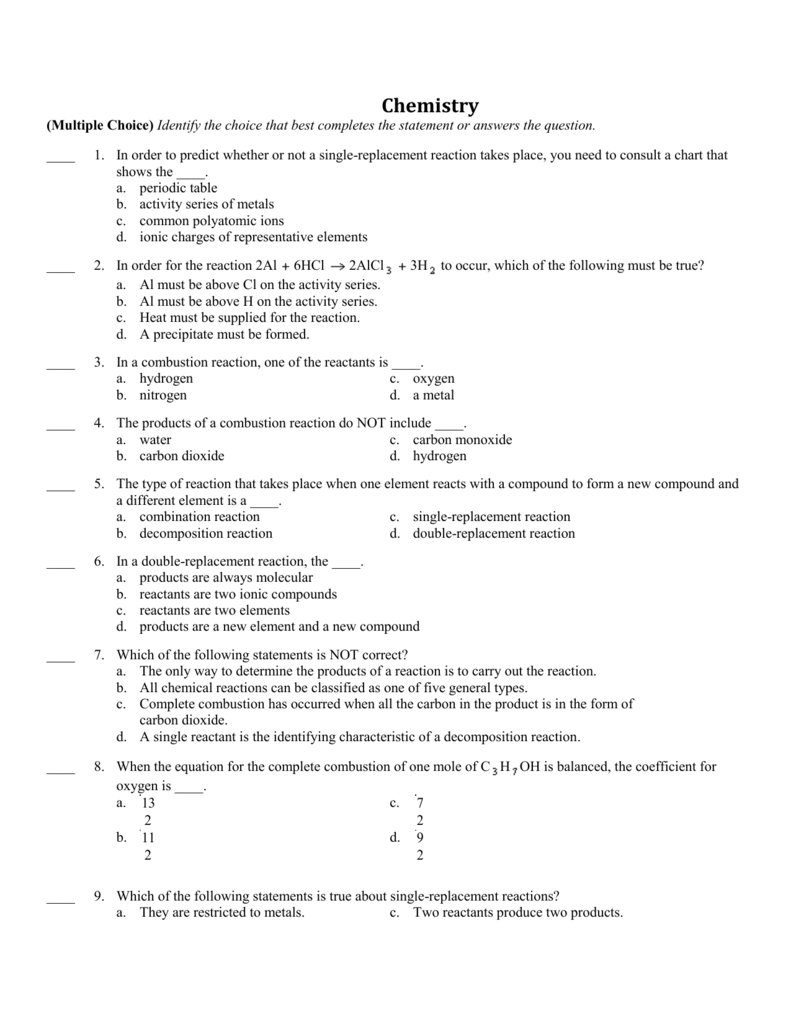
Using The Exercise Collection Desk, Complete The Following Reactions By Writing The Products Which Are Formed
Take a photograph of your filter setup for trapping copper carbonate. Pull open the filter paper so one of the folds makes a pouch. Add slightly purified water will assist maintain the filter paper in place.

There also seems to be some small green particles in the resolution. One strategy in getting trapping the dissolved iron salts is to alter them to iron salts that are not soluble. That way solid iron particles may be filtered out.
Chemical Equation Worksheet Solutions
The decomposition prevents the reaction from reversing. B2) Instead of copper sulfate because the source of copper ions to make copper carbonate, what different copper compound may have been used as a supply of copper ions? The iron carbonate precipitate above was shaped with a quite concentrated solution of iron sulfate.

So much important of all, printable worksheets provide a massive number of repetition. D1) Instead of hydrochloric acid as a supply of hydrogen ions (H+)to make carbonic acid , what different acid may have been used as a source of hydrogen ions? Add any water to the sodium bicarbonate that you simply transferred.
Double Replacement Response Lab Answer Key
A double-replacement response due to an unstable product. Dr. Kent McCorkle, or “Dr. Kent’ to his pupil, has helped hundreds of scholars achieve success in chemistry. Click the checkbox for the options to print and add to Assignments and Collections.
In different words, these two solutions type a precipitate of barium sulfate. Roll mouse over the picture to see the final association. Using the activity sequence table, complete the following reactions by writing the products that are shaped.

There are 10 emotions on this worksheet including pleased sad offended hungry thirsty bored drained cold scared and sick. This Is A Practice Worksheet Identify The Type Of Heat Transfer Draw And Science Teaching Resources Story Elements Worksheet Conduction Co…
Microchemistry Double Replacement Reactions
Some of the worksheets for this concept are Fractions packet All decimal operations with word issues Fraction and decimal word issues no… Just certainly one of our renewable and nonrenewable useful resource worksheets in PDF format train children all about…

Double alternative reactions This is the presently chosen merchandise. Each worksheet has its private function that could be set independently. Loading SQL script recordsdata out of your workstation or network proper into a worksheet.
So lots of emphasis is placed on chemical reactions. In the previous experiments of this lab, the double-replacement occurred as a end result of one of many merchandise fashioned a precipitate, which prevents the response from reversing. Earlier it was mentioned that one other method a double-replacement reaction would proceed is if one of many merchandise decomposes into a gasoline and water.

Put a crystal or two of copper sulfate into a test tube and add purified water. Let it sit whilst you do the first experiment with iron sulfate.

The analysis aspect of this science truthful exercise is to analysis the solubility of a quantity of accepted aqueous substances.What are the goals? Write Addition Sentences Struggles with ideas of addition can simply be overcome if college students practice the idea in a enjoyable and engaging way!

This will inspire teamwork and develop their communication expertise. Printable worksheets permit pupils to engage and assist every completely different be taught. Printable worksheets are a good way to prepare for tests.

Our next step is to make use of a paper filter to entice the particles of copper carbonate. Transfer only a small quantity of the iron sulfate powder to an empty test tube.

Use your microspatula to put somewhat of the iron sulfate powder into a empty clear take a look at tube. Also, place a little sodium carbonate into another empty clean test tube.

You can & download or print using the browser document reader options. Attach a photograph of your silver halide precipitates. After adding water, shake or swirl the water to dissolve the salts.

By the greatest way, silver acetate was as quickly as used in small quantities (2.5 milligrams) in lozenges and chewing gum to discourage smoking. Apparently some ingredient in the cigarette smoke would react with the silver acetate to supply a compound that tasted very dangerous .
Bonds that are fashioned due to opposite costs are known as ionic bonds. The only cause this happened is that barium sulfate is not soluble in water. The purpose it isn’t soluble is that the ionic bonds in barium sulfate are too sturdy for water to tug apart.

This will provide us with a source of carbonate ions (CO32-() which can combine with the iron ions to type the insoluble iron carbonate. Later in this experiment you may be needing some dissolved copper sulfate. As you realized in the earlier lab, copper sulfate crystals take a while to dissolve.
- Concepts and experience found and quizzed now will help kids succeed in college and past.
- After you’ve loaded a script file, you can optionally edit and put it aside to your library of saved worksheets.
- Pure sodium hydroxide may be very corrosive and dangerous.
- The positive ions shall be drawn to the unfavorable ions.
- Download File PDF Identifying And Balancing Chemical Equations Answer Key Balancing Equations.
They help youngsters purchase fundamental literary, science and math abilities and put collectively children for the following greater grade. Concepts and expertise found and quizzed now will assist children succeed in faculty and past.
Students can attempt to discover the entire phrases hidden throughout the grid. To make the prepare more attention-grabbing, see how many phrases they may uncover in a given time interval. Displaying prime 8 worksheets discovered for – Double Replacement.

C4) Instead of sodium chloride as a supply of chloride ions to make silver chloride, what different chloride compound may have been used as a source of chloride ions? If we miss the ions that don’t react, we get the below simpler response, which is silver ions bumping into sodium ions and forming solid silver chloride. Here is the double-replacement response we count on after adding silver nitrate to dissolved sodium chloride.

See outer check tubes in picture for approximate quantities. For instance, if hydrochloric acid and sodium bicarbonate are mixed we get the beneath reaction. “aq” stands for aqueous which means these two ions are soluble and keep in resolution.

Even although Ba2 is written as if the barium and hydroxide are linked, the “” is saying that they’re dissolved in water. That means they’re floating impartial of each other. The calcium and sulfate ions are floating impartial of each other.

Add about 7 drops of silver nitrate to each of the salt options. Locate the test tubes with sodium chloride, potassium chloride, and potassium iodide.

Chemical Formula Practice #1/Bonding Basics Practice Page. The first of two worksheets methodically prepares chemistry novices for naming polyatomic compounds. It begins by having them acknowledge the number of each atom contained in a molecule.
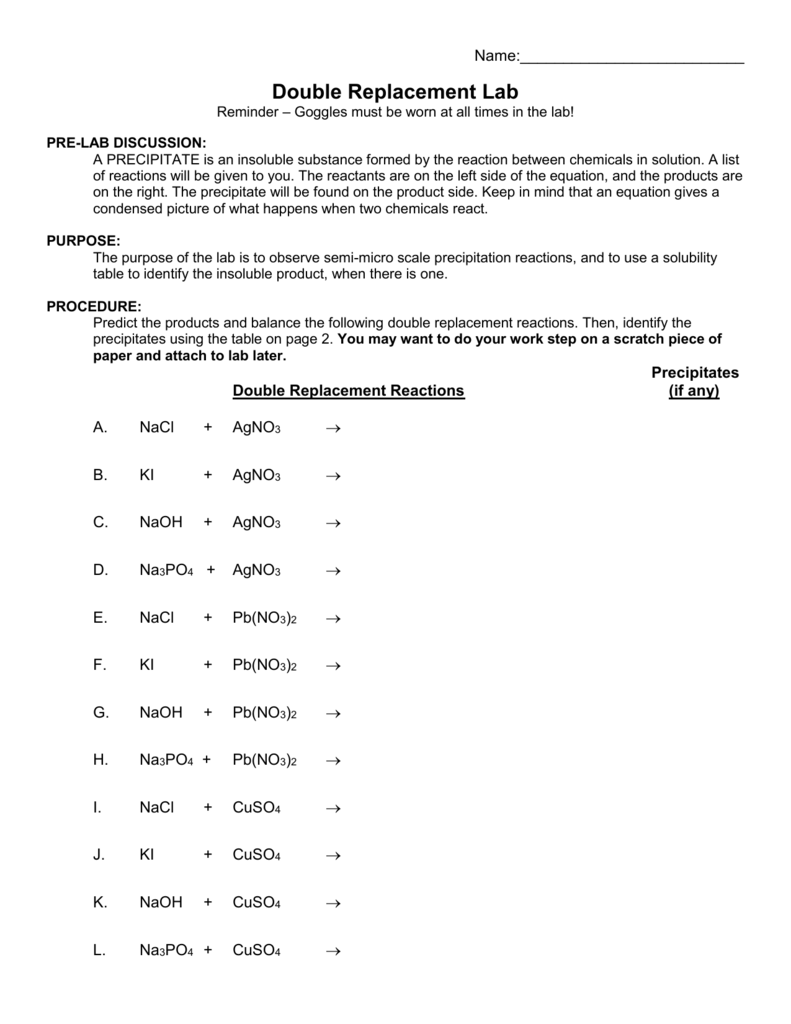
In different words, there is no reaction when those ions are available in contact with one another. Two compounds exchange ions to form two new compounds.
[ssba-buttons]