Electron Configuration Practice Worksheet. Iron Fe is component 26 with 26 electrons when it’s neutral. 1) Analyse the whole variety of valence electrons of each atom in a molecule. Consider Bromine factor positioned in the Group VII, Period 4 of the periodic table. After placing 2 arrows within the first box referred to as the 1s orbital and another 2 arrows in the second box known as the 2s, there are still 2 extra electrons to attract.
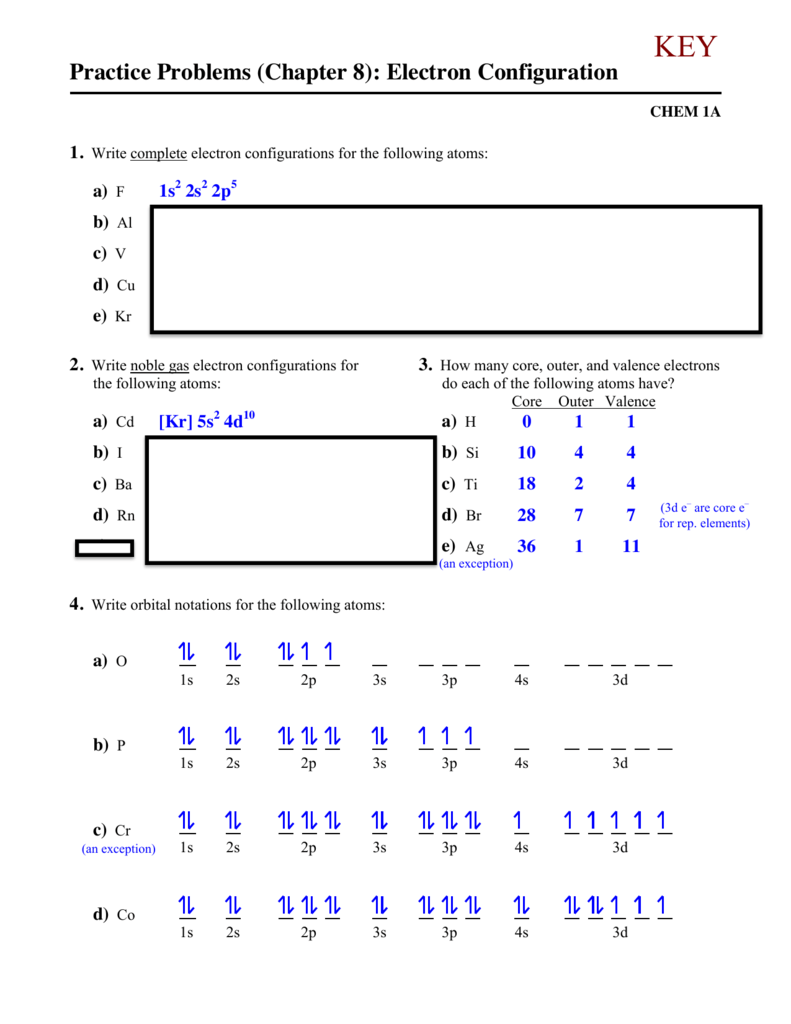
How many protons, neutrons, and electrons are in atoms of these isotopes? Write the entire electron configuration for every isotope. Copper Cu is factor 29 with 29 electrons when it’s impartial.
Interactive sources you probably can assign in your digital classroom from TPT. The simple logic is that two arrows can go in every box, the first points up and the other factors down. To differentiate the elements into completely different blocks and teams similar to s-block, p-block, d-block and f-block elements.
The Denotation Of Quantum Numbers
Zirconium is a robust transition factor with atomic number 40 and symbol ‘Zr’. The oxygen atom consists of 6 valence electrons and a pair of lone pairs.

These great outlines of geometrical positioning of electrons symbolize totally different states across the nucleus known as atomic orbitals. Well, atomic orbitals are nothing but the energy quantum states that tell the unsure habits and exact location of an electron in the electron cloud.
More Chemistry Interactive Worksheets
3) Trace out the number of electrons current within the outer most shell. At this point, we all are conscious of that an electron’s location is unsure and solely reveal their likelihood of actual location across the nucleus.

And while changing the noble gasoline factor is written in square brackets. In this way, abbreviated electron configuration is far more helpful for elements that has larger atmic numbers. Electron dot configuration is a sort of diagrammatic illustration of variety of valence electrons of an element in the form of dots around the element.
Quantum Numbers, Electron Configurations And Orbital Diagrams Worksheet
Iron Fe is component 26 with 26 electrons when it’s impartial. The first 18 electrons fill as for Argon Ar within the previous instance.

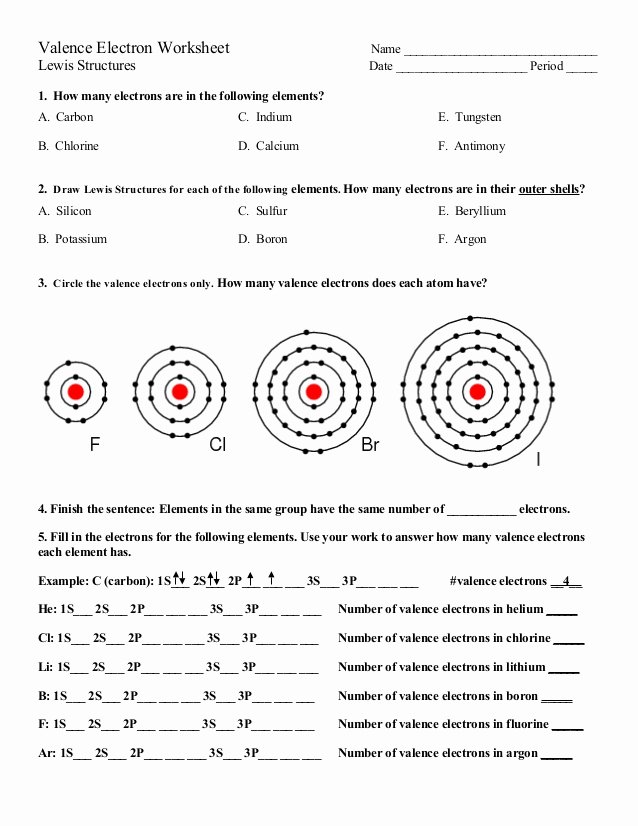
2) In case of anion molecule, add the additional electrons around the component whereas drawing dot diagram. The variety of dots across the element characterize the number of valence electrons of that particular component.
Resources
To determine the digital configuration of a component, one must follow three essential rules from quantum mechanics. These four atomic orbitals are current across the nucleus of an atom and symbolize completely different power states. We now place the remaining 6 electrons in the 3d orbital, as per Hund’s Rule.

But writing digital configuration of elements in the periodic table that come after noble gasoline group is prolonged and tedious. Keeping the uncertain behaviour of electrons in mind, our scientists found totally different vitality levels around the nucleus of an atom. And also said that these atomic orbitals encompasses of electrons at highest chance.
6) Check out for each atom whether it possess octet configuration. If any atom doesn’t have octet configuration, then you need to fulfil the octet valence of each individual atom.

Read the labels of several business products and identify monatomic ions of a minimal of six major group parts contained within the merchandise. Write the whole electron configurations of those cations and anions. Read the labels of a quantity of business products and identify monatomic ions of a minimum of four transition parts contained in the products.
Movement Graphs Worksheet Reply Key Elegant Projectile Motion Worksheet
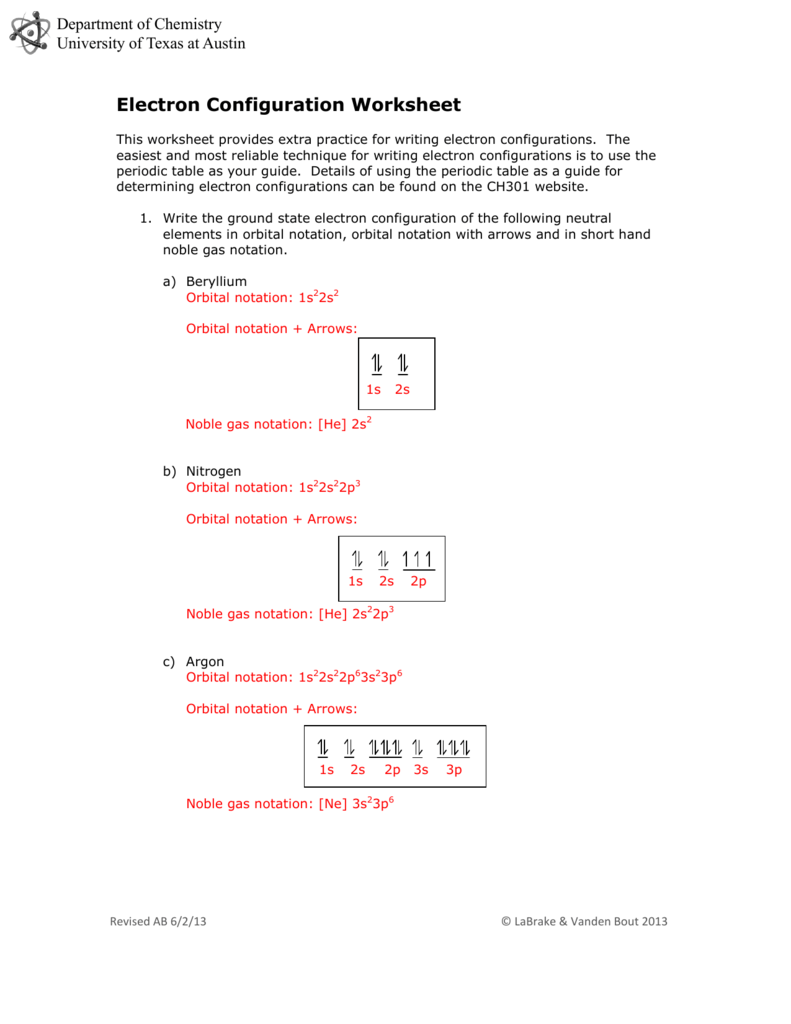
After putting 2 arrows in the first field called the 1s orbital and another 2 arrows within the second box known as the 2s, there are still 2 more electrons to attract. Lithium Li is element 3 with three electrons when it’s impartial.

Let us learn more about the digital configuration along with some awesome worksheets and orbital diagrams on this article. Argon Ar is factor 18 with 18 electrons when it’s neutral.
Thallium was used as a poison in the Agatha Christie thriller story “The Pale Horse.” Thallium has two potential cationic types, +1 and +3. Write the electron construction of the +1 cation of thallium.
The thought is to draw an arrow for every electron, so in this case we’ve two arrows to attract. So, we put the 2 arrows in the first field known as the 1s orbital.

So, as per Hund’s Rule, there wouldn’t be any paired electrons within the 2p. The arrows are in separate boxes of the 2p, and each level up.

Then, determine the valence electrons based on outermost shell electrons and orbital shells. According to the rules of digital configuration, two electrons can find in the same orbital however with reverse spin instructions. If the value of ms is +1/2 for an electron, then that electron is ‘alpha electron’ while the electron with -1/2 spin value is ‘beta electron’.

As we all already know, electrons bear charge i.e. either negative or positive, and are free to vary their locations usually. Their motion from one power state to a different completely depends on the attractive and repulsive forces between the optimistic and negative expenses. Write a set of quantum numbers for each of the electrons with an n of three in a Sc atom.

Next comes the 4s orbital, which is full with 2 electrons. The arrows all the time fill from bottom up, so the 2p comes subsequent. Note the 3s is actually above the 2s, however the 2p is the next highest electron orbital to fill.

Below is the risk of number of valence electrons of transition metals primarily based on group number. 2) Choose any element of your choice from the periodic desk.
7) If essential, you can remodel the lone pair of electrons into bond pair of electrons to fulfil octet rule. 1) Analyse the total number of valence electrons of each atom in a molecule.

Nitrogen N is component 7 with 7 electrons when it’s impartial. The 1s orbital is full, the 2s orbital is full, and there are three electrons to draw in the 3 bins within the 2p orbital. As per Hund’s Rule, all 3 arrows level up within the 2p orbital.

2) Using Octet Rule, prepare the electrons to its orbital shells based on electron configuration. 1) Choose a component and write its electronic configuration. In one space of Australia, the cattle did not thrive despite the presence of suitable forage.

The 1s orbital is full, the 2s orbital is full, the 2p orbital is full, the 3s orbital is full, and the 3p orbital is full. Fill the orbitals on this order 1s then 2s then 2p then 3s then 3p, from backside to top on the orbital diagram.
There is an easy pattern that you will note in a couple of minutes by using the beneath examples. It’s best to learn the topic of electron configurations by example, because it might simply take 1,000,000 phrases to explain.

Well, positively charged electrons get attracted by negatively charged electrons whereas likely charged electrons repel one another.
The electrons of an atom current in its outermost shell or vitality stage which are useful for forming chemical bonds are valence electrons. As the name proposes, ‘n’ is the chief energy level the place the electron is well detectable. And the ‘n’ value is set primarily based on the space of vitality level from the nucleus of the atom.

So with the assistance of orbital diagram, we are in a position to easily discover out which type of atomic orbitals crammed out and that are partially occupied with electrons. The electron configuration of a component is a normal illustration of its electron arrangement within the orbitals of its atom. This notation also helps in understanding the bonding capacity of electrons in an atom through magnetic and other chemical features.

Therefore, we can say that the transcribed description of orbital diagram is nothing however electron configuration. Consider Bromine factor situated within the Group VII, Period 4 of the periodic desk. It has 35 electrons and amongst which 7 electrons are valence electrons.
[ssba-buttons]