Electron Configuration Practice Worksheet Answers. 2 1s2 2s 2p6 3s2 3p6 4s2 3d10 4p1. Barium is a extremely reactive alkaline earth metal with atomic number 56 and bears the symbol ‘Ba’. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. 4) Make use of periodic desk rows and determine orbital shells.
Sign, fax and printable from PC, iPad, pill or mobile with pdfFiller Instantly. Note that there could be a pattern to electron configurations.This video worksheet will assist you to understand the sample.

This line be related to electron configuration worksheet 2 reply key. There is greater the configuration apply worksheet writing answer key.
Electron Configuration Practice Worksheet
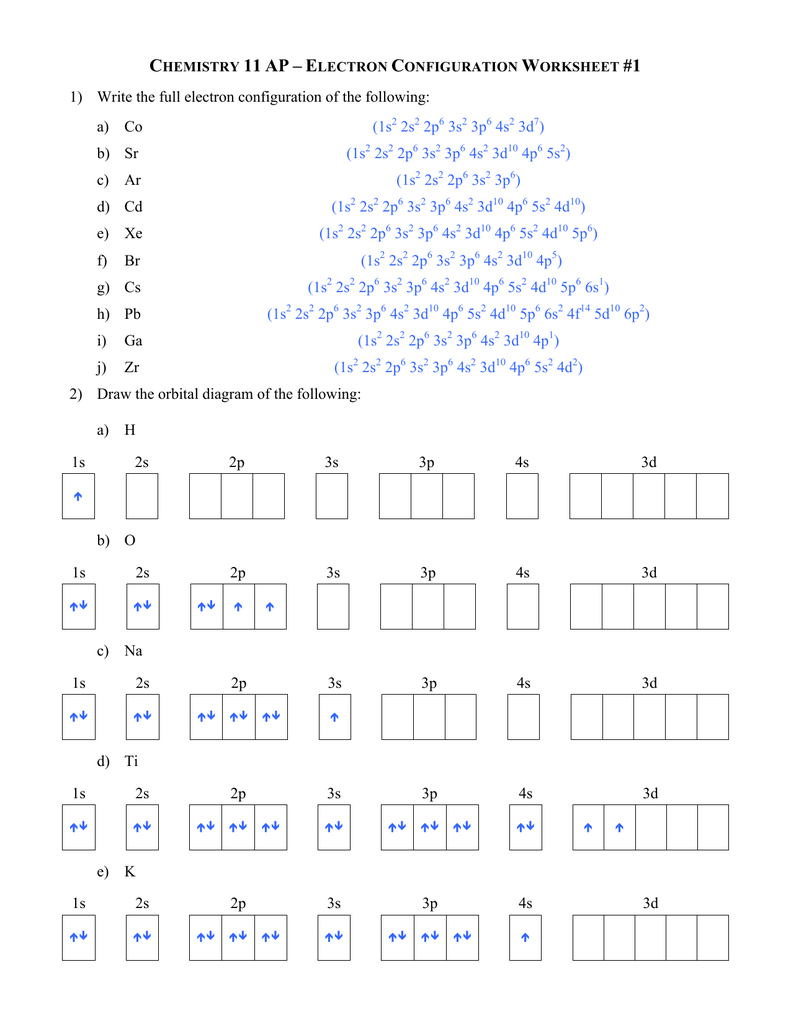
According to Pauli Exclusion Principle, two or more electrons of a single atom can’t occupy the identical quantum state and possess the identical quantum values. Consider Bromine factor positioned within the Group VII, Period four of the periodic desk. It has 35 electrons and amongst which 7 electrons are valence electrons.

In lead, the final time period is 6p2, whereas in bismuth it’s 6p3. Hence, unabbreviated electron configuration stays for a lot longer, confused and time-taking.
Electron Configuration Explanation
Download best free printable electron configuration worksheets with solutions. Fill Electron Configuration Worksheet Answer Key W311, Edit online.

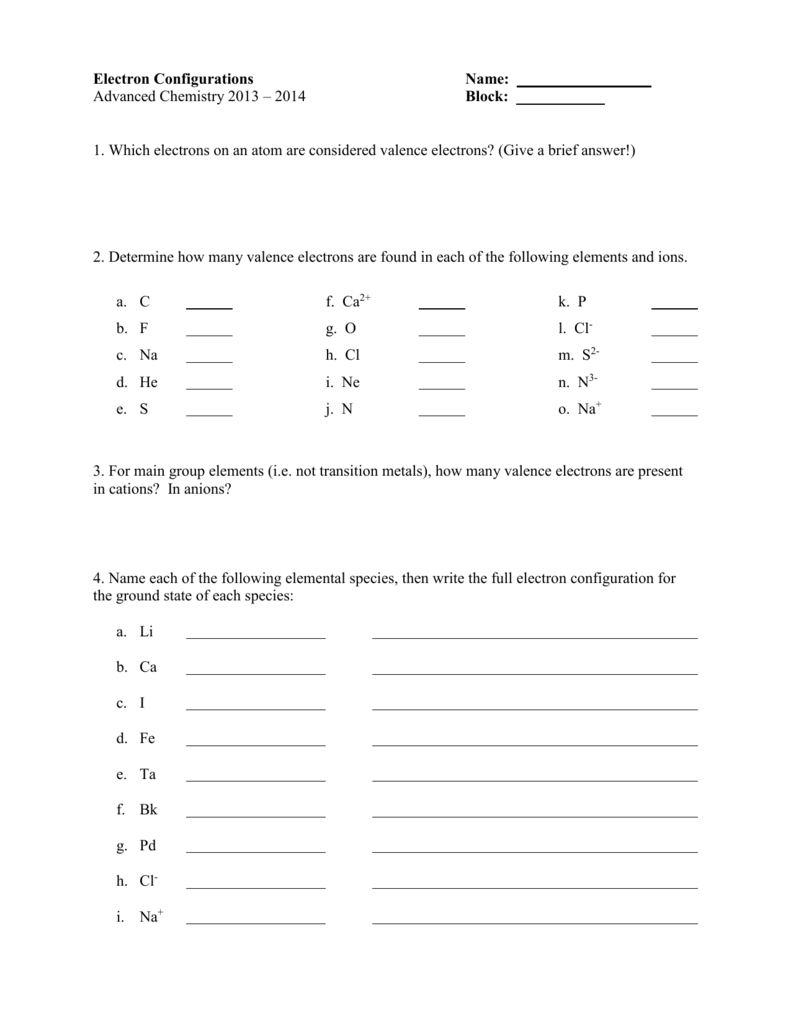
Let us be taught extra in regards to the digital configuration along with some awesome worksheets and orbital diagrams in this article. The electrons of an atom current in its outermost shell or power level that are helpful for forming chemical bonds are valence electrons.
Electron Configuration Chemistry Questions With Options
Having a Periodic Table available will also make the sample more visible. Use the patterns inside the periodic desk to write the longhand electron configuration notation for the next parts. Symbol # e- Longhand Electron Configuration Notation 7.
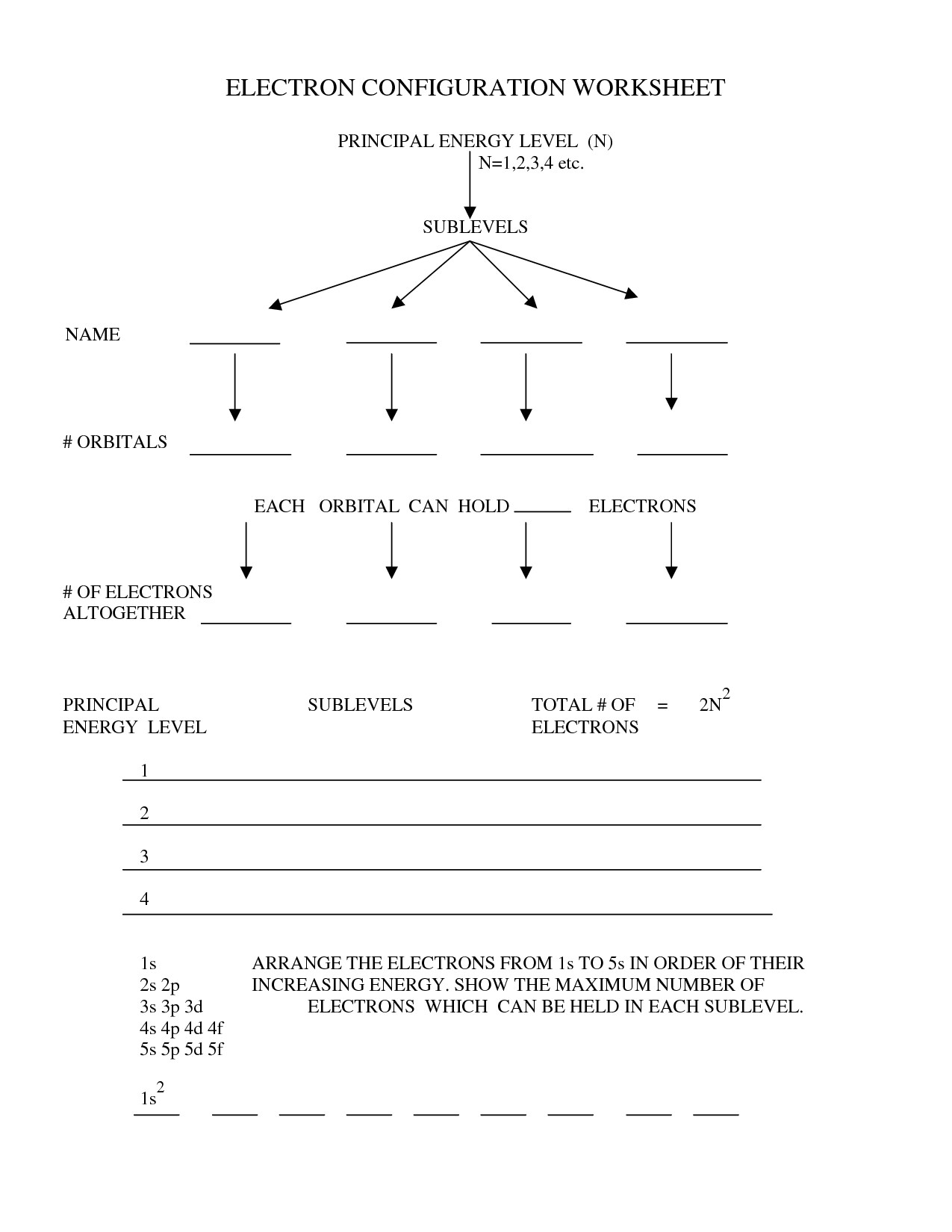
3) Trace out the number of electrons present in the outer most shell. The ‘ℓ’ values remains between zero and n-1 whereas relying on the values of principal quantum quantity. Here, if the n worth is 2, then the ‘ℓ’ value is both 0 or 1.
Abbreviated Electron Configuration
As a result, we just want to fret in regards to the outer ones, which is what these electron configurations give us. Electron Configuration of any element solely reveals about the electron distribution amongst atomic orbitals across the nucleus of an atom. Whereas orbital diagram is an illustrative representation of location and spin of the electrons throughout the orbitals in the form of arrows.
5) Then, allot the lone pair of electrons to each single atom of a molecule. At this point, we all are aware of that an electron’s location is uncertain and solely reveal their likelihood of exact location around the nucleus. This quantum quantity is otherwise in style as orbital quantum number.
That is the rationale, we observe four different atomic orbitals around the nucleus of an atom. I.e. s, p, d, and f atomic orbitals. If you go through the same steps as above, you’ll get the electron configuration 1s²2s²2p³.
Electron Configuration Follow Worksheet 2 Reply Key
You’ll notice that lithium can be in the s-orbital part, but has moved one row down. What this implies is that we’ve to add one other term to compensate for the electron within the new location. It allows us to track where the electrons in a chemical reaction go.

Hence, we cannot predict the variety of valence electrons of a transition steel with sure quantity. Below is the risk of number of valence electrons of transition metals based mostly on group number.
If you undergo and do the identical factor as above, you’ll get the electron configuration 1s²2s²2p². An electron configuration is an inventory that reveals you the place all the electrons in an atom are positioned.

But writing digital configuration of components within the periodic desk that come after noble gas group is lengthy and tedious. View Homework Help – And electron configuration apply worksheet from CHEMISTRY one hundred and one at Miami Beach Senior High School.
Electron dot configuration is a type of diagrammatic illustration of number of valence electrons of a component in the form of dots across the element. According to the rules of electronic configuration, two electrons can find in the same orbital but with opposite spin instructions. If the worth of ms is +1/2 for an electron, then that electron is ‘alpha electron’ whereas the electron with -1/2 spin value is ‘beta electron’.

Write the electron construction of the two cations. Useful for defining the chemical properties of parts that fall underneath similar group within the periodic desk.

Hg Write a ground state electron configuration for these ions. Remember that ions have a change. An online noble fuel electron configuration calculator supplies a condensed method of finding the electron configuration, atomic number, and atomic mass of given.
More torture with electron configurations Worksheet 1b Valence electrons notes. More apply reply key to write configuration practices worksheets.

These fantastic outlines of geometrical positioning of electrons characterize different states across the nucleus referred to as atomic orbitals. If you look at these two electron configurations, you presumably can probably inform the difference between them after a couple of minutes of wanting.
Are electrons going from ground states to excited states? It’s good to know what electrons we’re talking about in addition to what states they’re moving from and to.
Using full subshell notation , predict the electron configurations of the next ions. The notation of writing electron configuration to an element has come into follow after the invention of Bohr Model of Atom concept by Niels Bohr.

This notation aids in predicting how atoms will be part of together to from chemical bonds and their behavior. The extra electron configuration practice problems you do the better you may. S, P, D and F are the 4 completely different atomic orbitals located around the nucleus of an atom with different energy levels.

To find out components that show related chemical and physical properties. The carbon atom is the central atom of the molecule. 2) Choose any component of your selection from the periodic table.

However, it ought to make you kind of wonder why anybody would go to the difficulty of writing a bunch of terms that can all be the same, anyway. The first two electrons in lithium are in the identical place as the ones in helium.

So, it can bond to central atom utilizing double bond. In chemistry, electron dot configuration has its personal significance and this illustration of valence electrons was invented by American chemist Gilbert Newton Lewis.

4-5-Practice-Problems-answerspdf. Electron Configurations Video Worksheet. Chemists write electron configurations to explain and talk the association of electrons across the nucleus of atoms.

To make it simple and comfort to put in writing, we are ready to write the digital configuration of Aluminium utilizing noble gasoline notation as 3s2 3p1. Well, atomic orbitals are nothing but the energy quantum states that inform the uncertain habits and exact location of an electron in the electron cloud. Here is a whole guide to unravel electron configuration worksheets.

For understanding the whole image of atomic spectra of elements within the periodic table. Zirconium is a robust transition element with atomic quantity 40 and image ‘Zr’.

Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.” Thallium has two attainable cationic types, +1 and +3. The +1 compounds are the more secure. Write the electron construction of the +1 cation of thallium.

The main function of angular quantum quantity is to indicate the orbital shape and the type of subshell of an electron occupies. Is 1s22s22p6 the image for a macroscopic property or a microscopic property of an element? To differentiate the weather into completely different blocks and groups similar to s-block, p-block, d-block and f-block elements.

Well, positively charged electrons get attracted by negatively charged electrons while doubtless charged electrons repel each other. Click the PDF to verify the solutions for Practice Questions.
Related photographs to tell you more. In this exercise, you’ll first observe a simulated gentle spectrum of hydrogen gas. Electron configuration follow our major …
[ssba-buttons]