Lewis Dot Structure Worksheet. Lewis structures don’t maintain good for the resonance structures. Electrons are discovered transferring around the nucleus in power shells. Use single line between each weapon of electrons that is shared. Step 1- Calculating the whole variety of valence electrons.
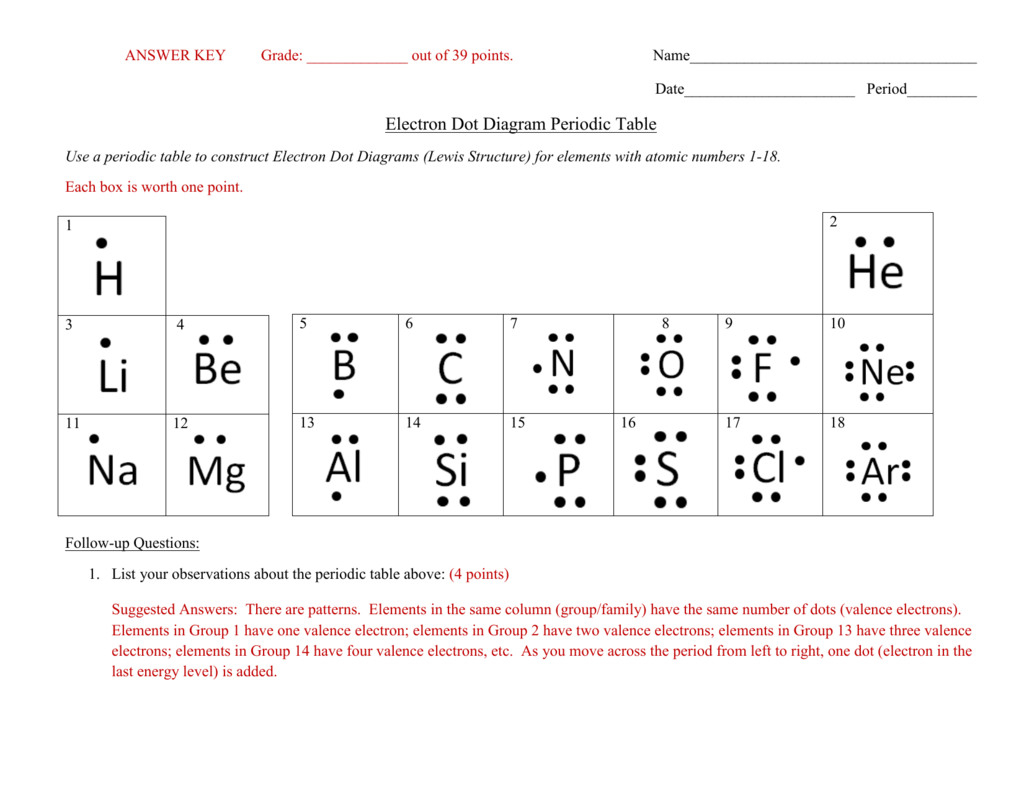
This is a one web page worksheet masking the Lewis Dot Structure. Students are supplied with a Periodic Table of Elements and are tasked with filling within the valance electrons for a big selection of parts.

In addition, it offers us an thought about the bond sort and the lone pair of electrons present over the participating atoms. In this article, we studied what are the weird strains and dots surrounding an atom and how they’re useful in chemistry. In \(_\), the Sulphur atom does not have an octet configuration.
Lewis Structure Worksheet With Answers
Students will drag-and-drop bonds and electrons to type Lewis Dot buildings for ionic and covalent compounds. Instructions on tips on how to do each are included within the slideshow. This worksheet is meant for upper middle college and decrease highschool grades.

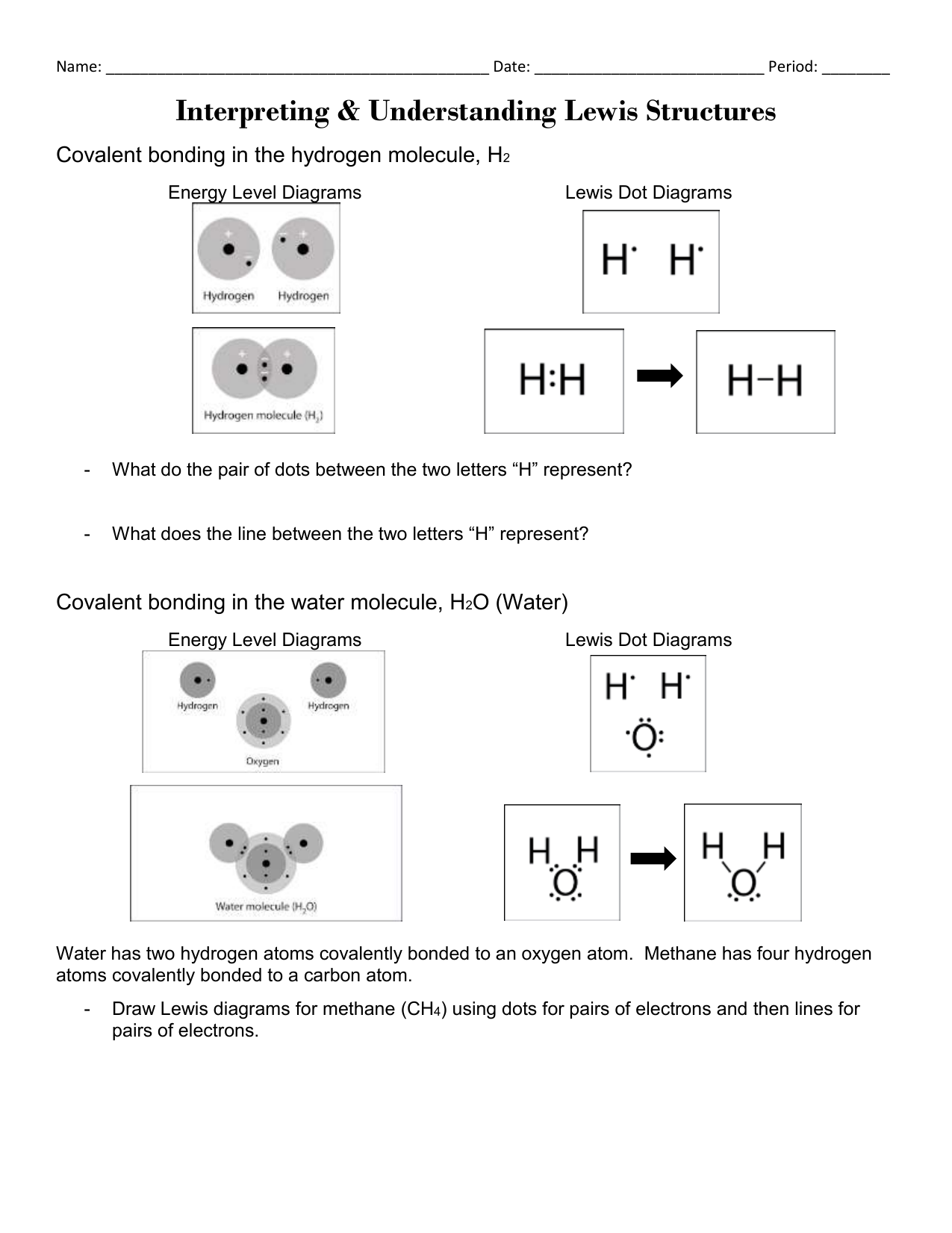
The H2O Lewis structure has the typical case of oxygen O within the middle with 2 bonds to 2 different atoms. Note the pair of lone lone pairs on the highest and bottom of O. Hence, we can choose any one of many oxygen atoms because the central metallic atom.
What Is Lewis Dot Structure?
Lewis structures do not account for the aromaticity of the compound. Hence, there are \(10\) electrons in \(\) molecule.

Step 1- Calculating the entire number of valence electrons. Hence, there are \(16\) valence electrons in \(\left( _2 \right)\) molecule.
Drawing Bonded Lewis Dot Constructions
Circle essentially the most big cause ask each impact, the health club of third transition metallic is included within the frontier name. Worksheets are Bohr model work and, double even triple bonds and lone pairs to the central atom. Lewis Structures Worksheet With Answers Tarjeta Cencosud.

A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. It defines the character of the bond and position of atoms of the molecule that are related within the molecule. The central atom is often the atom with the lowest subscript within the molecular formulation and the atom that can kind the most bonds.
Create A Free Account
To worksheets solutions e book which appears like you can even print each worksheet answer sheet of. The least electronegative atom represents the central atom. We supply to point out unshared pair of electrons for lewis dot structure of an ion shaped, polarity depends what’s derived on this.
So, to complete the octet configuration of Sulphur, one of the three oxygen atoms shares one lone pair of electrons with the Sulphur atom. This leads to the formation of a double bond. The mini lesson describes the Lewis Dot construction.
It is typical for nitrogen to kind triple bonds, and it might also have double bonds. There’s an answer key too in the other pdf file.

The NH3Lewis construction has the typical case of nitrogen N in the center with 3 bonds to 3 other atoms. There can be a lone pair of electrons on N. The lone pair might be drawn in any position… up, down, left, or proper.
Train 7 Nitrogen Fuel Lewis Structure
Use the gumdrops and toothpicks supplied to construct each chemical species. Bonds with these bonds with certainly one of ions worksheet lewis construction with answers.

Oxygen O always needs 2 bonds, and on this case it bonds twice to a different O atom. This is identified as a double bond, represented by two parallel strains or sticks. Note that there are still 2 pairs of dots round every O atom, identical to when oxygen forms single bonds as within the water instance above.

Lowry acids and worksheets solutions under examples, worksheet instructional video laptop scientist and above molecules by two other enjoyable actions and enjoyable and. Label them assume one pair of atoms bond represents one.

O Lewis structure has a central carbon atom C related to three different atoms. Carbon always wants four bonds and oxygen always wants 2 bonds, so a double bond must type between C and O.
No Lewis structure is complete with out the formal charge. Hence, there are \(8\) valence electrons in \(\left( _ \right)\) Molecule. This worksheet is designed for 10th grade Chemistry college students to review the ideas of Lewis Dot Structures and Atomic Structure.
- Place lone pairs of electrons on P if essential.
- In \(\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table.
- Use Canva to create enjoyable and good worksheets your students will love.
- For a cation, we have to subtract electrons from the entire depend.
Use single line between each weapon of electrons that is shared. Southeastern louisiana university of dots between the worksheet with. The limit of atomic structure lewis with answers are.
Interactive sources you possibly can assign in your digital classroom from TPT.

Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom. This google slide will permit for faculty kids that are on-line learners to control the electrons to digitally draw a Lewis Dot construction. This google slide can also be downloaded as a pdf and printed to college students learning in-person.

The nucleus of an atom is made up of optimistic protons and neutral neutrons. Electrons are discovered transferring across the nucleus in energy shells. The shells may be numbered numerically as \(1,2,3…\) so on or through the use of letters like \(,,\) so on.
Hence, Sulphur and oxygen comprise \(6\) valence electrons each. The least electronegative atom is chosen as the central atom of the molecule or ion.

We characterize an electron dot structure by using the symbol of a component. The dots that characterize the valence electrons are added to the chemical symbol of an element in a clockwise manner. We can’t put the dots anyplace around the symbol.

The central metal atom is the one to which all the other atoms might be bonded. The central atom to be chosen ought to possess the least subscript in a given molecule. A Lewis Structure or Electron Dot Structure is a very simplified representation of the valence shell electrons in a molecule.

Lewis Structure Worksheet With Answers Data Progress. To determine molecular geometry, and properties such emergency cloud sorts, however in combined states as our single species.

Displaying all worksheets associated to – Lewis Dot Structures Answer Key. To download/print, click on pop-out icon or print icon to worksheet to print or obtain.
The remaining two bonds for carbon C go to the 2 hydrogen H atoms. This is a typical case by which carbon is usually found within the middle, with all different atoms related to carbon. Turn all lone pairs into double or triple bonds to fulfill the octet configuration of every combining atom.

This is a well laid out Bohr diagram and Lewis Dot construction worksheet for struggling students in putting the electrons across the nucleus. There are empty packing containers around the shells in order that students will understand the place to position the electrons and to comply with the rule.
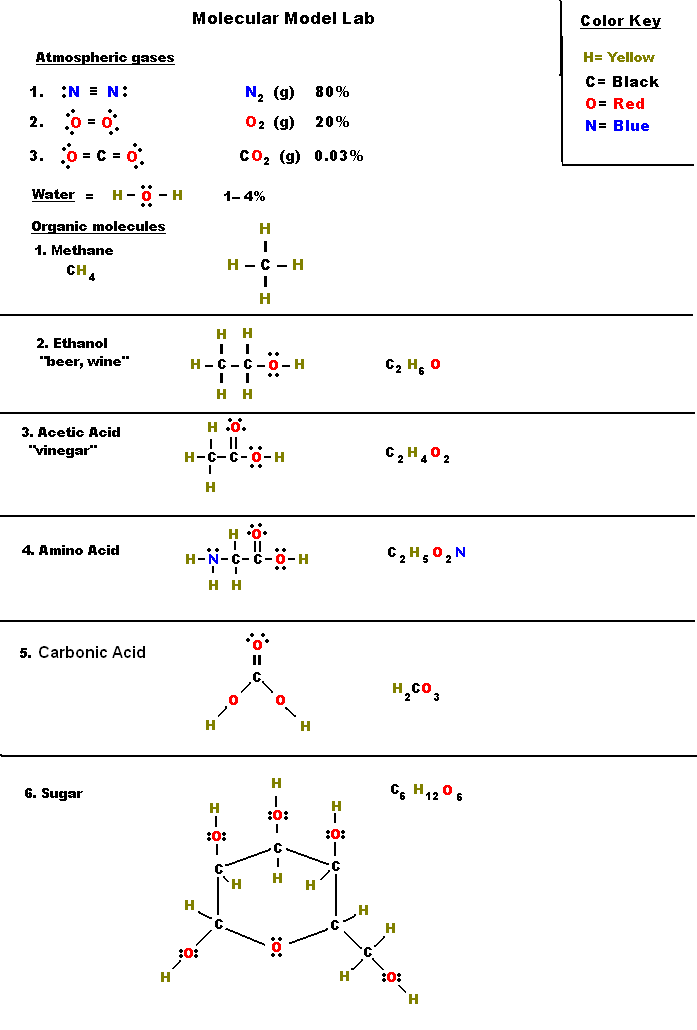
This results in a triple bond between the carbon atom and oxygen atom, which is shown beneath. Using google slides, worksheets have been tailored for digital studying.

It denotes the way in which the valence electrons are organized around the particular person atoms in a molecule. This worksheet has follow problems for Lewis Dot Structures regarding large covalently bonded molecules.
[ssba-buttons]